Reptile medicine, and by extent surgery, is becoming more common in veterinary practice. Reptile ownership in the UK is higher than ever (Tedds et al., 2020) and, as a result, reptiles are far more likely to present to general practice. In the UK, reptile ownership is far from a new trend, with tortoises having been kept as pets here for over a century; however, not all owners are able to meet husbandry requirements adequately, resulting in a number of injuries and illnesses.
Unless a veterinarian has a particular interest in reptile medicine, they are unlikely to be performing advanced surgical procedures such as ovariosalpingectomy, but general practitioners may be called on to perform minor procedures, such as digit, tail or hemipene amputation, abscess debridement or lump removals. For all these procedures, a thorough knowledge of reptile anaesthesia and what sets it apart from small animal anaesthesia is essential to gain the best outcomes for your reptile patients.
Reptilia anatomy and physiology
The vast number of species within the class Reptilia make generalisations of anatomy and physiology necessary for this article; however, species-specific differences should be investigated prior to attempting anaesthesia.
Thermoregulation
Thermoregulation is a very important aspect of anaesthesia in reptiles due to the fact they are ectothermic. This means that their body temperature matches the environmental temperature and when their body temperature is lower, so is their metabolism (Mans et al., 2019). Being ectothermic has implications for drug metabolism during induction, maintenance and recovery. Therefore, reptile patients should always be kept at their preferred optimum temperature zone (POTZ), which is unique to each species. In general, this is considered to be 20 to 25°C for temperate species and 25 to 30°C for tropical species (Mans et al., 2019).
Reptile patients should always be kept at their preferred optimum temperature zone (POTZ), which is unique to each species
Cardiovascular anatomy and physiology
Snakes, lizards and chelonians have a unique cardiovascular anatomy and physiology as they only have three heart chambers – two atria and one ventricle (Sladky and Mans, 2012). The ventricle is incompletely divided by an intraventricular septum that allows the mixing of deoxygenated blood with oxygenated blood, but most importantly, it allows shunting of blood from pulmonary to peripheral circulation and vice versa (Mans et al., 2019).
Snakes, lizards and chelonians have a unique cardiovascular anatomy and physiology as they only have three heart chambers – two atria and one ventricle
This physiology is of particular importance when using inhalational anaesthesia because if the patient diverts blood from the pulmonary circulation, inhalational agents cannot be exhaled and, therefore, continue to provide anaesthesia even if the inhalational agent has been ceased. Cardiac shunting can also affect circulating oxygen concentrations and systemic blood pressures which affects anaesthetic monitoring.
Inhalational agents cannot be exhaled and, therefore, continue to provide anaesthesia even if the inhalational agent has been ceased
The hepatic first pass effect is also particularly important in lizards, crocodilians and chelonians. This occurs when drugs are injected into the hindlimb where venous blood flow drains into the ventral abdominal vein, which then leads to the liver (Mans et al., 2019). Drugs that are metabolised or excreted by the liver are then broken down before taking effect, resulting in significantly reduced bioavailability of drugs when compared to administration in the forelimbs (Kummrow et al., 2008).
Respiratory anatomy and physiology

Although all reptiles lack a diaphragm, respiratory anatomy and physiology differs widely between reptilian species. Respiration requires the use of pelvic and pectoral muscles, and patients may need to be ventilated under anaesthesia due to relaxation of these muscles. Spontaneous respiration is controlled by hypoxia and hypercapnoea (Mans et al., 2019), although reptiles tolerate hypoxia well (Sladky and Mans, 2012). This fact is useful to understand when attempting to recover the patient as maintaining the patient on piped oxygen following a procedure will likely result in the patient staying apnoeic. Once the procedure is concluded, the patient should be ventilated with room air as needed until spontaneous ventilation begins (Figure 1).
Analgesia
Appropriate analgesia should be provided as part of the anaesthetic plan and, ideally, as a premedication to ensure the patient is analgesed during any procedure. Multimodal analgesia is encouraged and a number of opioid, non-steroidal anti-inflammatory and local anaesthesia options are available (Balko and Chinnadurai, 2017).
Induction and intubation
An extensive review of injectable agents for anaesthetic induction by Mans et al. (2019) is available to help the clinician decide on their induction of choice.
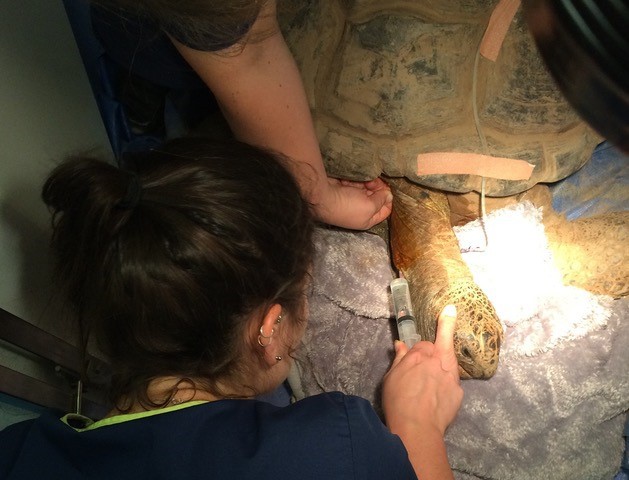
Inhalational induction can be achieved, although it can take a considerable amount of time and requires high concentrations of inhalational agents, which results in environmental contamination (Mans et al., 2019). Injectable anaesthesia is preferred due to the speed of onset and the ability to deliver titratable volumes. Ideally, injectable anaesthesia is provided intravenously (Figure 2) rather than intramuscularly as large volumes of injectable agents can be painful and often take longer to take effect than intravenous administration (Mans et al., 2019).
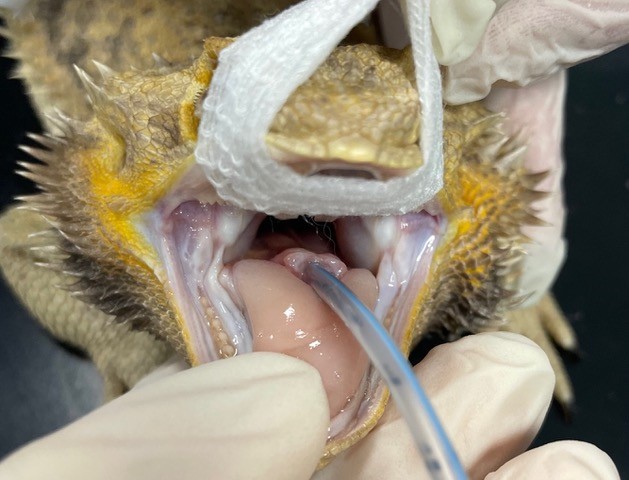
Intubation of reptiles is essential as all reptiles require intermittent positive pressure ventilation (IPPV) under anaesthesia
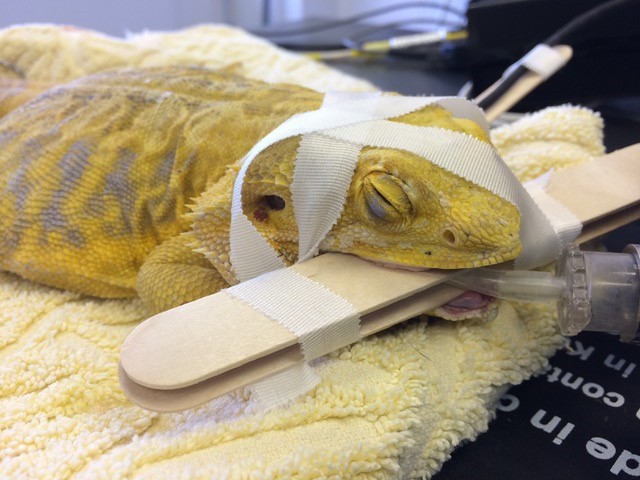
Intubation of reptiles is essential as all reptiles require intermittent positive pressure ventilation (IPPV) under anaesthesia. Once the patient has been appropriately induced, the glottis can be located at the base of the tongue in most reptilian species (Figure 3) (Olsson and Simpson, 2018). As the patient takes a breath, the glottis opens and the endotracheal tube can be advanced. An uncuffed tube is recommended, and tubes can be secured in any number of ways to ensure they stay in place for the duration of anaesthesia (Figure 4) (Worrell, 2019).
Maintenance
To maintain an end tidal carbon dioxide (ETCO2) between 10 and 25mmHg, all reptiles should be provided with IPPV once they are at a surgical plane of anaesthesia (Mans et al., 2019). Depth of anaesthesia can be measured by muscle tone, withdrawal reflex and evidence of spontaneous respiration. Righting reflexes can be used in snakes and lizards, and palpebral reflexes can be used in species with eyelids (Sladky and Mans, 2012).
Minimum heart rate should be monitored with a Doppler probe over the heart or via an oesophageal stethoscope
Anaesthetic monitoring can be difficult as there is very little information about the vital parameters of reptiles under anaesthesia. However, minimum heart rate should be monitored with a Doppler probe over the heart or via an oesophageal stethoscope. A pre-anaesthetic heart rate should be obtained to compare to the values under anaesthesia if ranges have not been established for the species undergoing anaesthesia. Electrocardiogram (ECG) use has been reported in reptiles, but little information is available about parameters, even for commonly anaesthetised species (Mans et al., 2019). Indirect blood pressure measurement is difficult to obtain and has poor correlation to direct blood pressure measurements and therefore is not useful for monitoring (Chinnadurai et al., 2010).
Respiratory rate should be predetermined due to IPPV, and the use of a mechanical ventilator can provide accurate respiratory pressures and rates. ETCO2 should be measured by capnography with ventilation adjusted if there is evidence of hypercapnoea.
Recovery
Recovery time is based on several factors including body temperature and, therefore, metabolic rate, as well as the drugs administered to induce and maintain anaesthesia (Mans et al., 2019). Ideally, the patient should be kept in an incubator or vivarium at the POTZ. IPPV should be continued until the patient is spontaneously respiring, at which point the patient can be extubated.
Recovery time is based on several factors including body temperature and, therefore, metabolic rate, as well as the drugs administered to induce and maintain anaesthesia
Recovery times following isoflurane general anaesthesia have been shown to reduce following the administration of adrenaline in snapping turtles (Goe et al., 2016).
Round up
Reptile anaesthesia can be achieved to a good standard in general practice and can be incredibly rewarding for clinicians and patients alike. An understanding of the unique anatomical and physiological differences between reptiles and small mammals is essential for successful anaesthesia of reptilian patients.